

BASIC INFORMATION
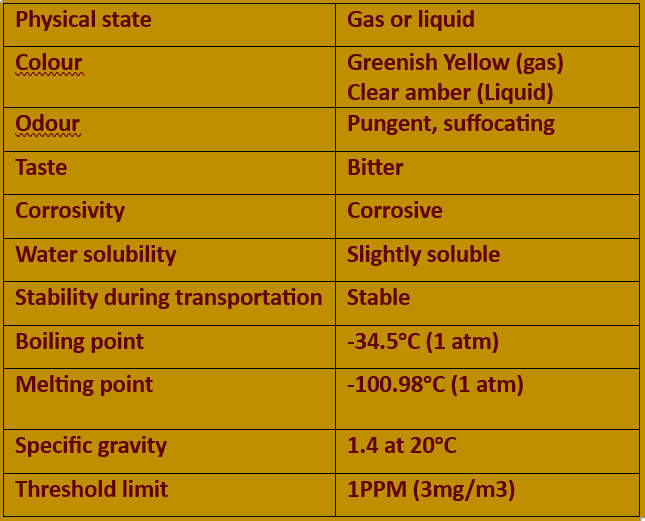
Pure chlorine comes in two forms: gas and liquid. Chlorine gas is easily liquefied under pressure. Typically, a commercial cylinder contains liquefied gas under pressure.
Chlorine gas has a disagreeable, sharp, pungent, penetrating odour. In airborne concentrations above 1000 parts per million (ppm) it has a greenish-yellow colour. In smaller concentrations it is colourless.
Chlorine gas is 2½ times heavier than air and tends to flow downhill and pool in lower areas. Wind and weather, however, will cause a chlorine gas cloud to disperse, spreading it in all directions, even uphill.
Liquid chlorine is a transparent, amber-coloured, oily fluid that is 1½ times heavier than water.
Liquid chlorine has a high compression ratio. The ratio of liquid to gas is 1 to 460, which means that 1 L of liquid chlorine expands to form 460 L of pure chlorine gas.
Chlorine will not burn by itself, but will support combustion.
Chlorine, in both gas and liquid forms, reacts with almost all chemicals, usually with a release of heat.
At high temperatures, chlorine reacts vigorously with most metals. For instance, a chlorine reaction can cause stainless steel to catch fire or melt.
Chlorine reacts with water or moisture in the air to form highly corrosive acids. Every precaution must be taken to keep chlorine and chlorine equipment moisture-free. Never use water on a chlorine leak.
There are basically 2 types of containers used in the supply and consumption of Chlorine. ‘Cylinders’ are small containers with a net carrying capacity in the range of 65 to 100 kgs. ‘Tonners’ are having net carrying capacity of about 900 kgs. Chlorine is filled in the container as liquid chlorine and remains under substantial pressure.
USES OF CHLORINE

Chlorine gas is mainly used as a disinfectant in:
Swimming pools
Water treatment plants
Sewage treatment
Community water supplies, including water used for irrigation
Chlorine is also used in:
Pulp and paper industries
Pool chemical products
Cleaning products
Mining processes
Bleach manufacturing Plastics manufacturing
PHYSICAL PROPERTIES
HAZARDS OF CHLORINE
Chlorine is corrosive. It can burn moist body surfaces such as the eyes, nose, throat, lungs, and wet skin because it forms harmful acids when it reacts with moisture.
Effects of Chlorine at various concentrations

STORAGE OF CHLORINE CONTAINERS

CHLORINE LEAK DETECTION

Chlorine leaks are usually confirmed using a standard ammonia test. This test is safe because it uses ammonium hydroxide (ammonia dissolved in water ) rather than pure ammonia. Chlorine reacts readily with ammonium hydroxide to form ammonium chloride, a relatively harmless compound. This reaction forms a white cloud, indicating a chlorine leak. The continuous monitors now required indicate chlorine leaks automatically, but the ammonia test is still useful for pinpointing the exact location of a leak.
MANAGING CHLORINE LEAKAGE
Take immediate steps to mitigate the situation as soon as there is any indication of presence of Chlorine in the air.
Chlorine leaks always get worse, if not attended promptly.
If the leak is major one, all persons in the path of the gas must be warned to leave the area and asked to act according to wind direction and higher than the leak. Since gaseous Chlorine is 2½ times heavier than air, it tends to lie close to the ground.
WATER SHOULD NEVER BE SPRAYED ON A CHLORINE LEAK. If it is done, will make the leak worse because of corrosive action of wet chlorine. Moreover heat from water increases evaporation rate of Chlorine.
If a container is leaking in such a position that chlorine is escaping as a liquid, the container should be turned so that Chlorine gas escapes. This reduces the hazards tremendously due to the following reason:
The quantity of the gas escaping from a gas leak is about 1/15th of the amount that escapes a liquid leak through the same size hole.
The severity of a Chlorine leak may be mitigated by reducing the pressure of the leaking container. This may be done by absorbing Chlorine gas (not the liquid) in a solution of caustic soda or soda ash or Hydrated lime slurry. Caustic soda is recommended as it absorbs chlorine more readily. If Hydrated lime is used, the slurry must be continuously agitated for chlorine absorption.
Availability of Safety kit for tonner/cylinder at site is also very helpful.
First Aid
Incase of Contact with Skin
1. Remove the victim to fresh air.
2. Remove the contaminated clothings.
3. Bring the victim immediately under safety shower for shower bath for atleast 15 minutes.
4. Don’t apply any ointment. 5. Get medical attention immediately.
Incase of Contact with Eyes
1. Remove the victim from the contaminated area.
2. Bring him to eyewash & shower unit.
3. Flush the eyeball with copious quantity of water for about 15 minutes.
4. Don’t apply for any ointment/chemical in the eyes. 5. Get medical attention immediately.
In Case of Inhalation:
If the patient is breathing, place him in comfortable position, keep him warm and at rest until a physician arrives. It breathing is difficult, administer oxygen if equipment and trained personnel are available.
Automatic artificial respiration is considered preferable to manual, but only when administered by an experienced operator.
Throat Irritation:
Drinking milk may help relieve the discomforts of throat irritation from Chlorine exposure.
Coughing:
A mild stimulant such a hot coffee or hot tea is often used for relief.
PERSONAL PROTECTING EQUIPMENTS
1. Airline Respirator
Airline respirators provided near the cooling tower area should be worn while connecting/disconnecting chlorine container.
2. B A Set
For tackling Chlorine emergency, B A Set is the most suitable respiratory protection. One should wear the B A Set from the safety cupboard kept in plant control room.
Non-Respiratory Protection
For moderate level of chlorine concentration, butyl or rubber clothing, gloves and hoods will provide good protection.
An effort must be made to seal the clothing so as to prevent the ingress of chlorine gas at the wrists, ankles and collar.
Gas tight positive pressure suit must be used in situation where the concentration of chlorine gas extremely high and if there is a necessity for a long period of exposure.
Notes:-
Avoid weather effects, store in well-ventilated heat deficient area.
Keep a solution of Caustic Soda/Soda Ash/Hydrated Lime Slurry in a tank ready for neutralization of Chlorine in emergency,
Keep a tonner/cylinder safety kit always handy.
Do not panic. Chlorine does not burn or explodes.
Avoid deep breathing when Chlorine is present in the atmosphere.


